diCELLa eCRF clinical trials

About diCELLa eCRF clinical trials
The diCELLa ecosystem is the software built by scientists, for scientists, with meticulous attention to every detail and real need.
diCELLa UNLIMITED is comprehensive software designed specifically for efficient management of clinical trials.
Our system offers all the necessary modules:
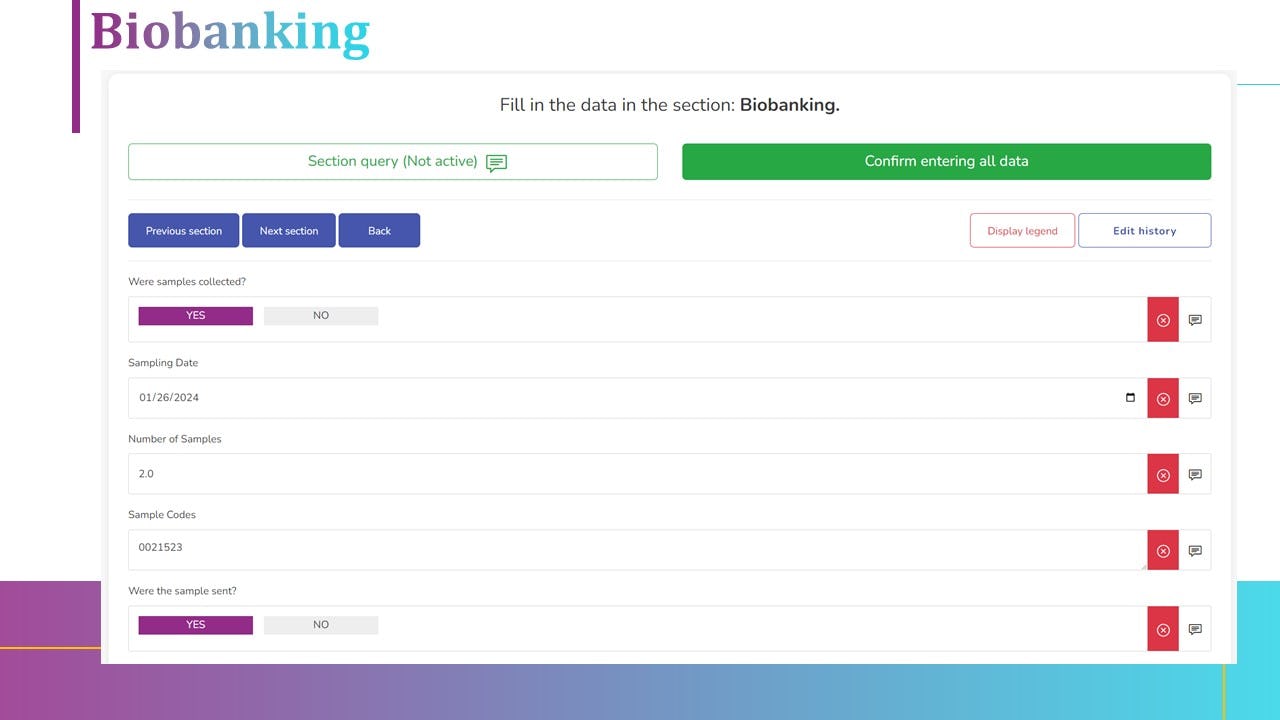
1. eCRF with remote monitoring module
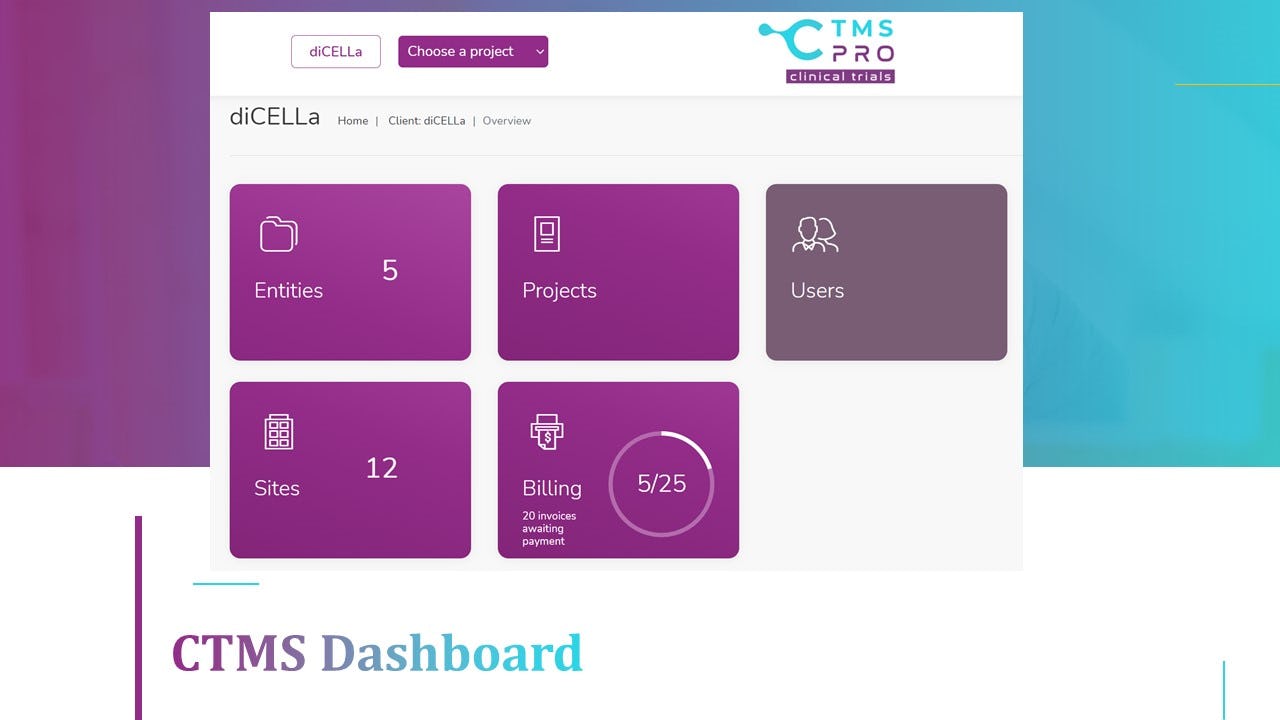
2. CTMS
3. IWRS/RTSM,
4. SAFETY: AE/SAE Tracking, Safety Management
5. CTMS interated with eCRF
5.a. patients management
5.b. sites management
5.c. SOP development
5.d. Agreements management
5.e. Team activities and working time/trip reporting
6. Query analysis
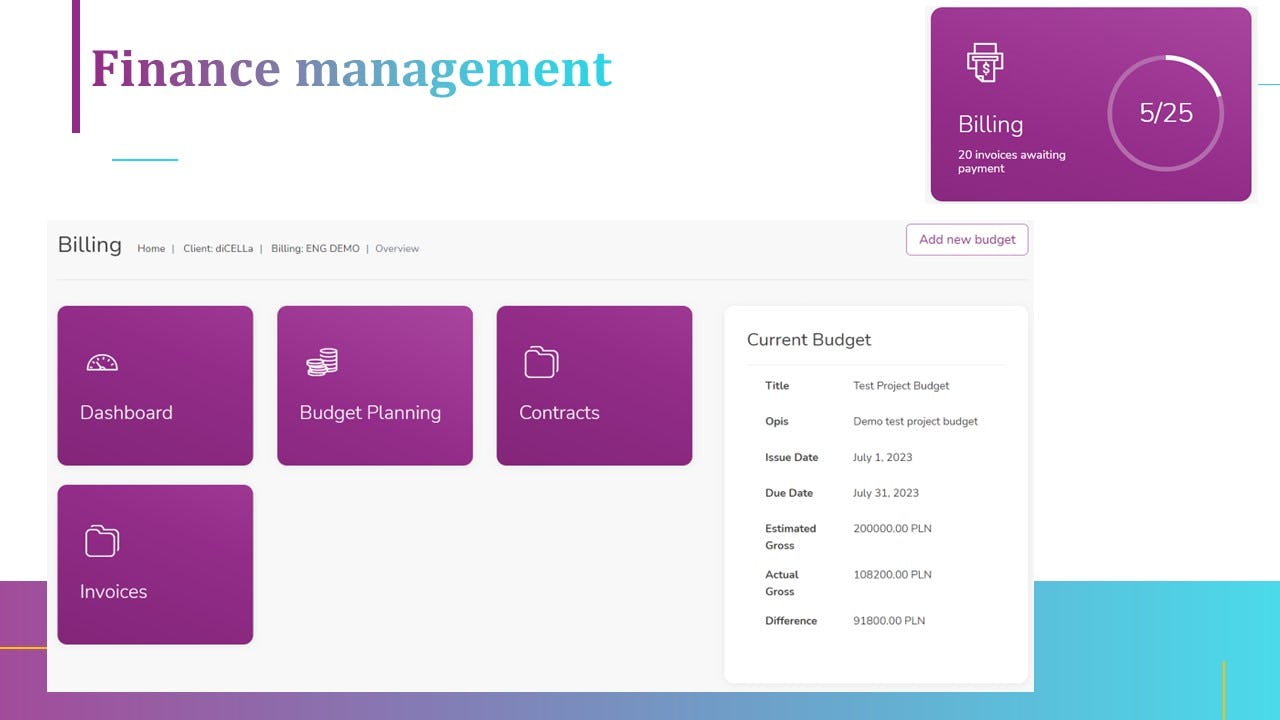
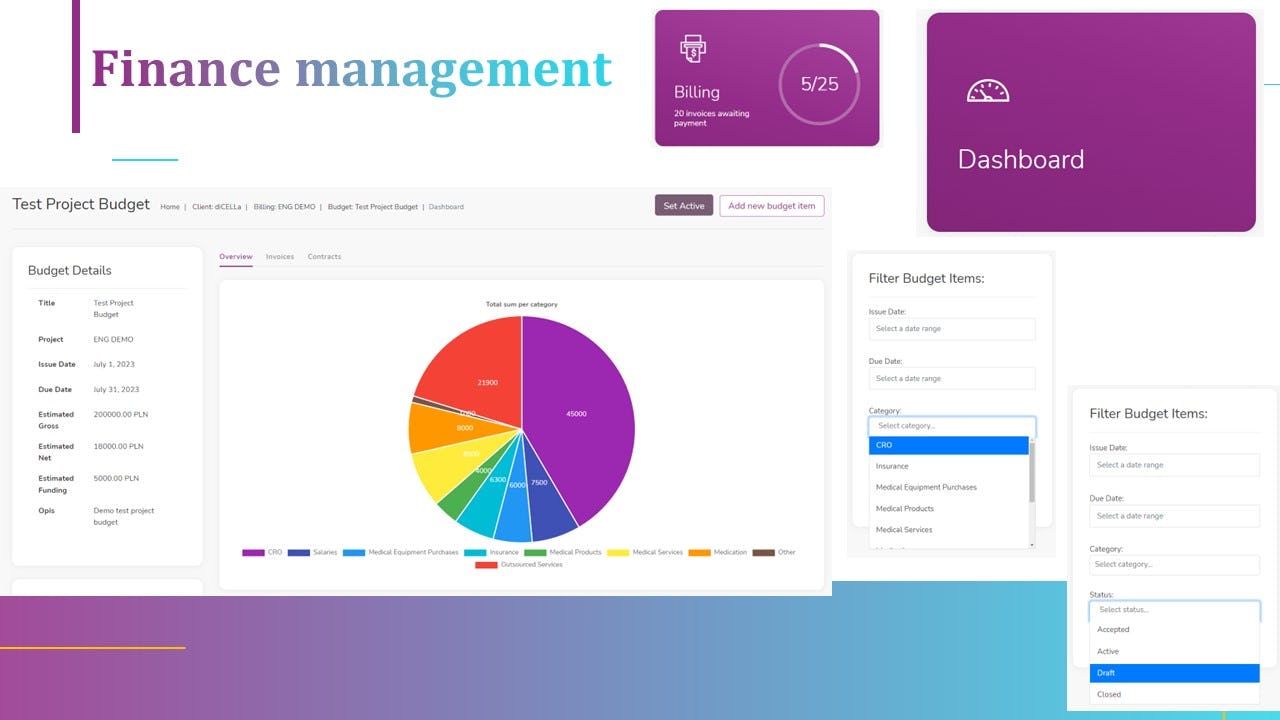
7. Finances, Budget Management and invoicing
8. eTMF/eISF
9. ePRO
10. Central Imaging Lab
Our tremendous success and honor come from our involvement in non-commercial clinical research, allowing us to collaborate with outstanding scientists and participate in ambitious projects. We believe that providing the right tools to medical personnel enables effective work and positively impacts team relationships.
Our integrated platform addresses the needs of all parties involved in clinical research: patients, investigators, study team, management team and sponsor.
Our passion is exploring the world and engaging in even the smallest projects, yet it truly ignites when we immerse ourselves in fervent research initiatives.
By combining knowledge in Mathematics, Computer Science, Medical Physics, and Medical Diagnostics, we have launched numerous exciting projects within our team. Originally, our specialization lies in the application of artificial intelligence in the analysis of medical and image data. Over time, as we delved into structured reporting in radiology and projects related to stem cells, we began collaborating with researchers conducting their own investigative projects.
The data in the application is secured with a patent-protected (EU, USA) method based on blockchain technology, which ensures their undeniability and authenticity.
Key benefits of diCELLa eCRF clinical trials
1. All-in-one platform
2. Payment model: configuration + maintenance
3. Training version
4. Training before SIV
Images
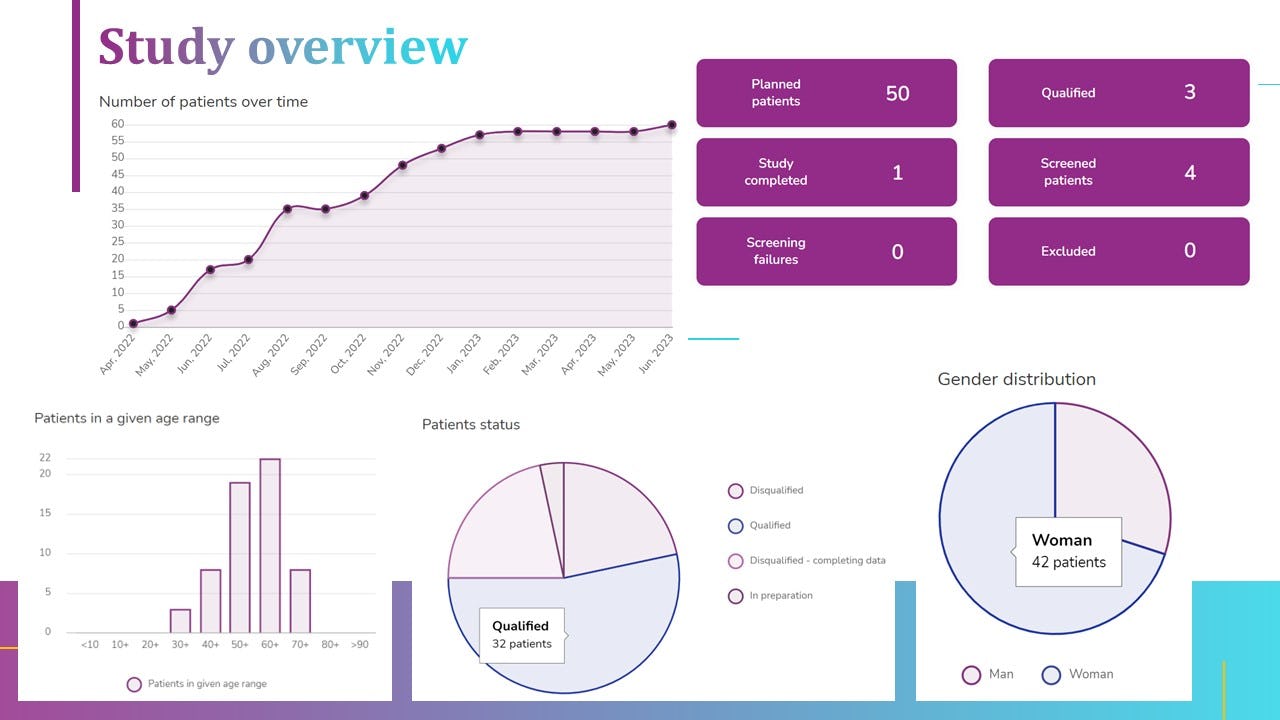
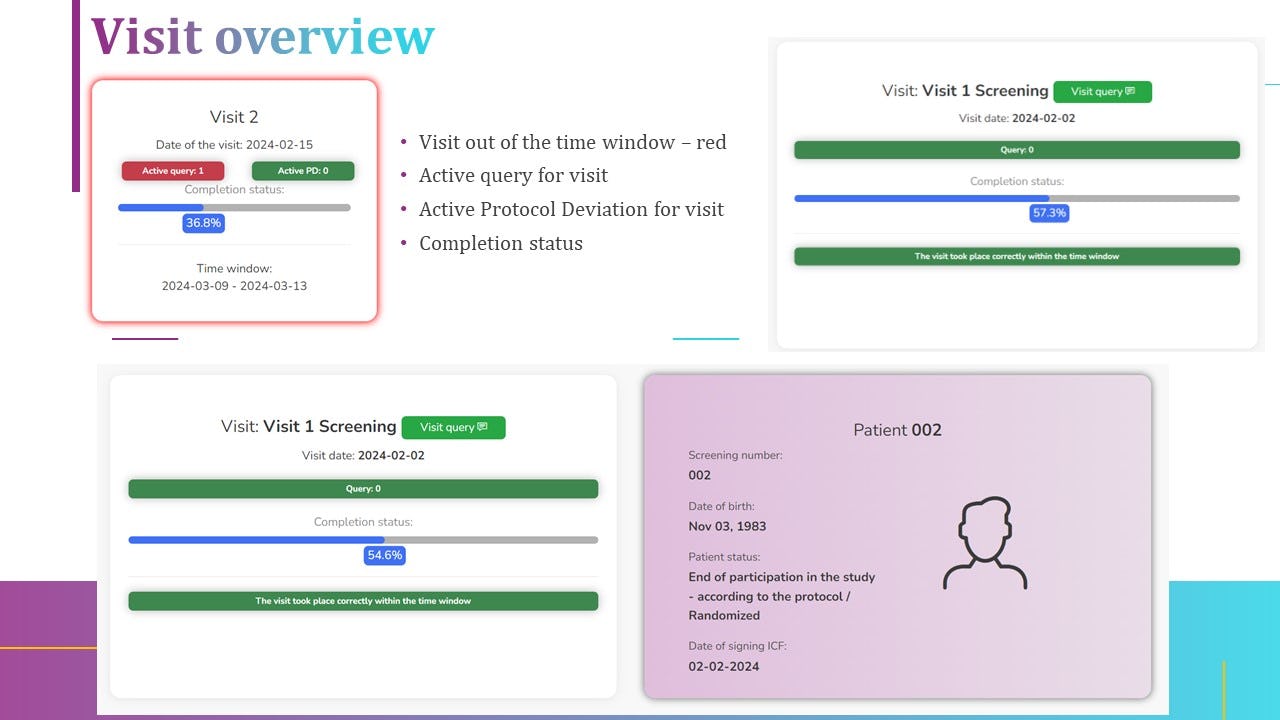
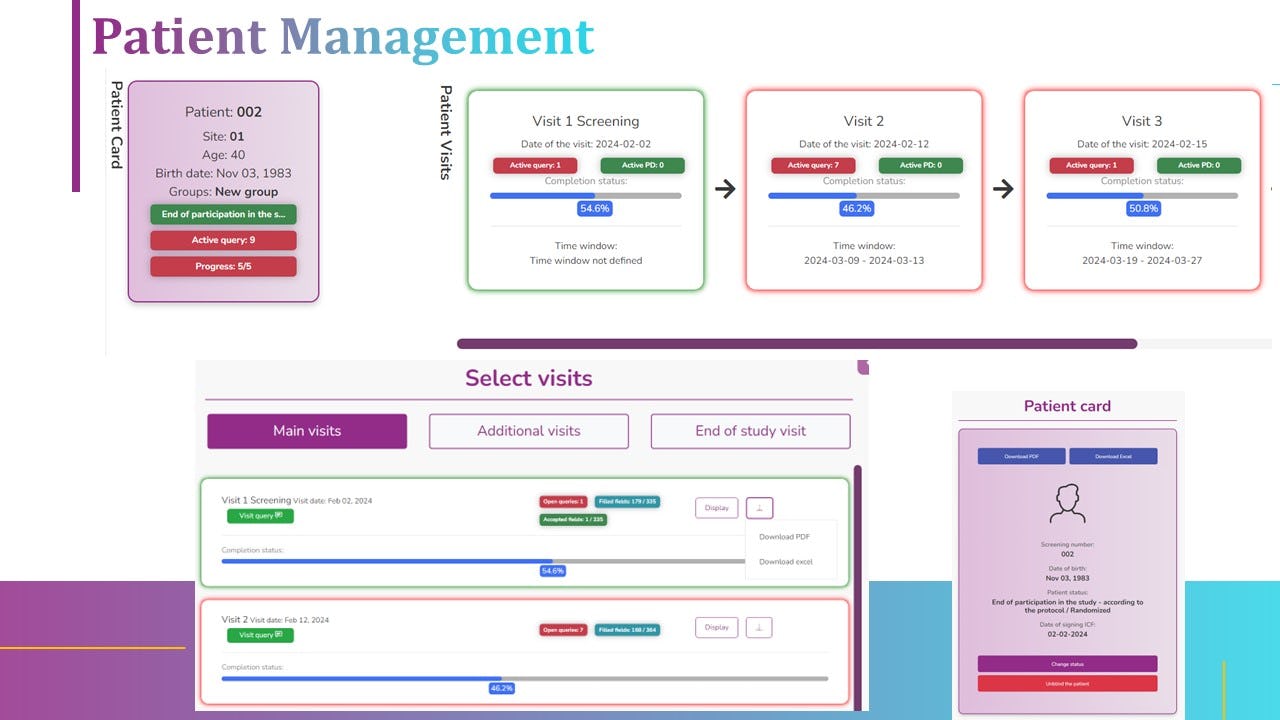
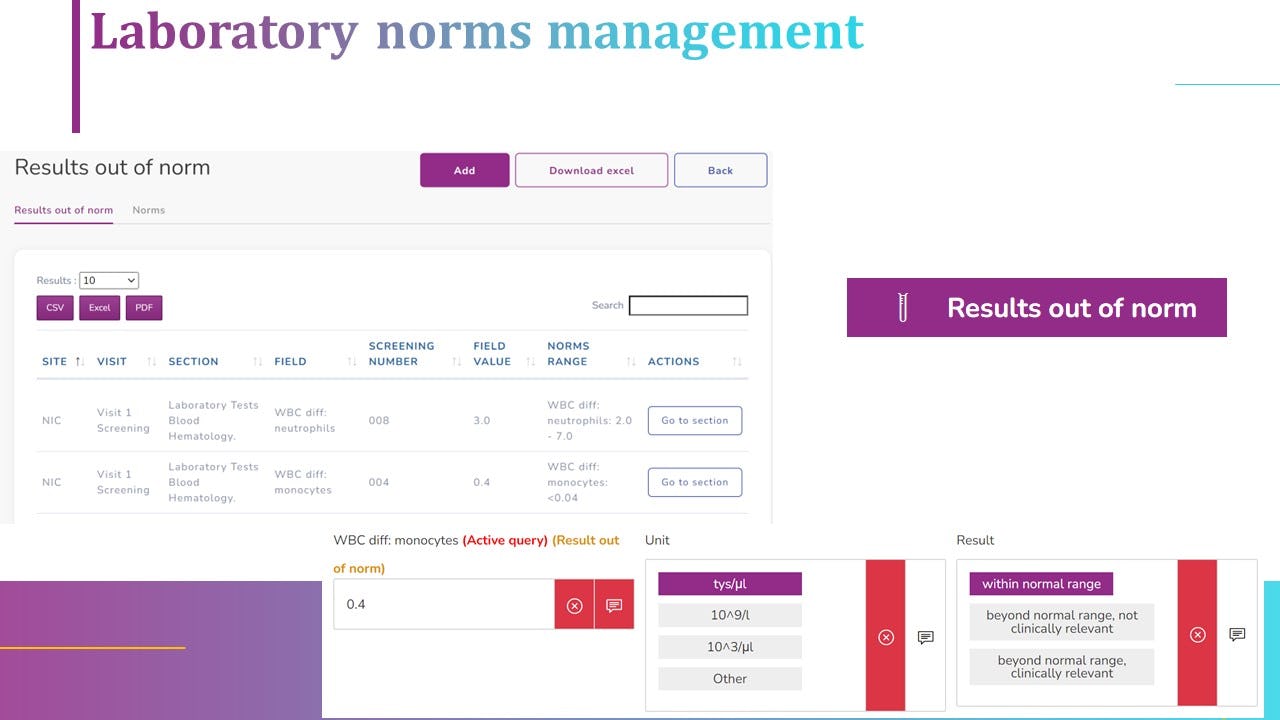

Not sure about diCELLa eCRF clinical trials?
Compare with a popular alternative

diCELLa eCRF clinical trials
Starting Price
Pricing Options
Features
Integrations
Ease of Use
Value for Money
Customer Service
Alternatives
Researchmanager – Clinical Research Suite

EvidentIQ eCOA

Flex Databases platform

TrialKit

Reviews

No reviews available
Software buyers need your help! Product reviews help the rest of us make great decisions.Already have diCELLa eCRF clinical trials?
diCELLa eCRF clinical trials FAQs
Below are some frequently asked questions for diCELLa eCRF clinical trials.Q. What type of pricing plans does diCELLa eCRF clinical trials offer?
diCELLa eCRF clinical trials offers the following pricing plans:
- Starting from: EUR 15,000.00/one-time
- Free Trial: Available
1. Customized Quotation: We provide a tailored quote based on your submission form. 2. Flexible Pricing: Our configuration costs are designed to fit your needs, depending on the complexity of your clinical trial, the number of visits, patients, and centers involved. 3. Transparent Estimates: We promptly send you a detailed quote for your review. 4. Seamless Onboarding: Once you accept our financial terms and sign the contract, simply provide us with your protocol and/or preliminary CRF. 5. Swift Setup: In just 5 days, we will prepare a customized eCRF for you. 6. Collaborative Customization: Engage in a collaborative process with us to fine-tune and align the eCRF with your workflow, ensuring it meets your team's unique needs. 7. Reliable Validation: Upon your approval, we conduct thorough validation, making sure your eCRF is ready to collect data seamlessly. 8. Ongoing Support: After the eCRF goes live, benefit from our continuous support with a straightforward monthly fee.
Q. Who are the typical users of diCELLa eCRF clinical trials?
diCELLa eCRF clinical trials has the following typical customers:
11–50, 51–200, 201–500
Q. What languages does diCELLa eCRF clinical trials support?
diCELLa eCRF clinical trials supports the following languages:
English, Polish
Q. Does diCELLa eCRF clinical trials support mobile devices?
diCELLa eCRF clinical trials supports the following devices:
Q. What other apps does diCELLa eCRF clinical trials integrate with?
We do not have any information about what integrations diCELLa eCRF clinical trials has
Q. What level of support does diCELLa eCRF clinical trials offer?
diCELLa eCRF clinical trials offers the following support options:
Email/Help Desk, FAQs/Forum, Phone Support, 24/7 (Live rep)
Related categories
See all software categories found for diCELLa eCRF clinical trials.








